Granted patent in Japan for Eyesiu's PEArlboost and Cyclosporin A lipid particle formulas
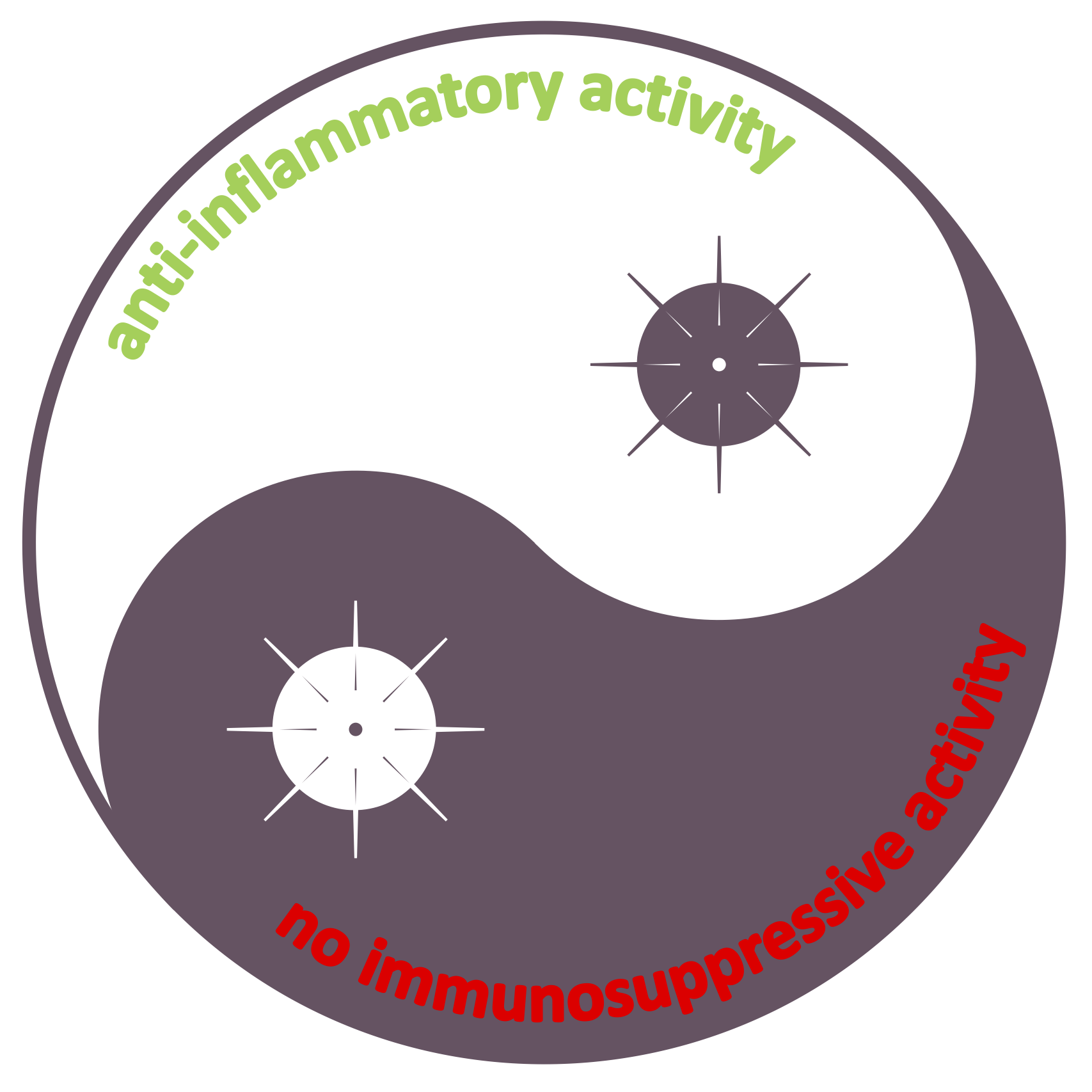
A patent was granted in Japan for Eyesiu’s PEArlboost and Cyslosporine A (CsA) lipid particle formulas. The granted claims cover an innovative PEGylated lipid particle spray that boosts the fast and sustained delivery of for instance the effective food supplement palmitoylethanolamide (PEA) and immunosuppressive CsA, which is likely the best compound known to date to reduce mortality in COVID-19. Also included in this set of allowed claims are all other related endocannabinoids from the group of acylethanolamides, such as 2-arachidonoylglycerol (2-AG), arachidonoylethanolamide (AEA or anandamide) and oleoylethanolamide (OAE), and other macrolides such as tacrolimus and sirolimus.
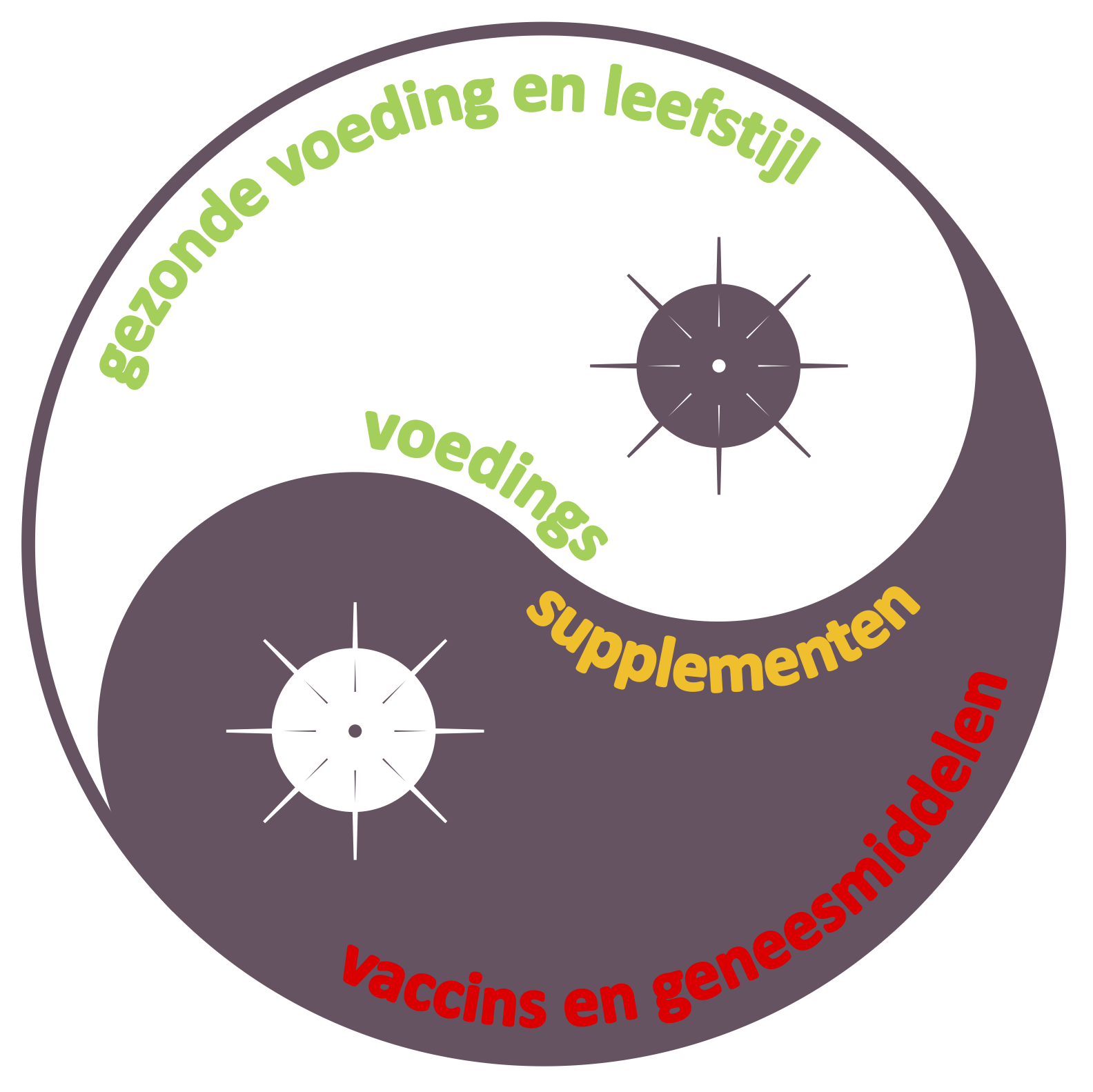
- Of special note is that at the start of the first wave of COVID-19 in Europe, Dr. Pieter J. Gaillard, founder and CEO of Eyesiu, already wrote a series of blogs in which he explained how in the immediate term and by using this innovative solvent-free and serum-stable nanotechnology, the existing medicines such as CsA for patients, and lipid-based formulations of food supplements such as PEA for consumers, can already help to prepare the body to combat COVID-19, the latter by providing fast and sustained targeted support for a healthy diet and lifestyle.
- See these blogs here (in Dutch about PEA), and here (in English about CsA and PEA), and here (about the PEA hypothesis).
- Meanwhile, on 15 October 2020, a first study was published in a Lancet article in which CsA in more than 600 patients with Corona showed that administration of this drug reduces the risk of death by 81%. These promising data were generated by a team at a Spanish hospital in Madrid, who analysed the records of 607 severe COVID-19 patients admitted in March and April during the first wave, when they were treated with one or more potential helpful drugs in critical cases, including CsA, glucocorticoids, tocilizumab, HIV antivirals, and hydroxychloroquine. In this study, CsA was the only drug that clearly reduced deaths (36 (14%) of the 253 patients who received CsA died, whereas in a closely matched comparison group 105 (almost 30%) of 354 patients who did not get the drug died).
- And on 3 December 2020, another key publication came out in the Journal of Internal Medicines detailing a pilot study that demonstrated again that Cyclosporine A plus low‐dose steroid treatment in COVID‐19 improves clinical outcomes in patients with moderate to severe disease. The authors from a hospital in Mexico concluded that CsA used as an adjuvant to steroid treatment for COVID‐19 patients showed to improve outcomes and reduce mortality, mainly in those with moderate to severe disease. The data analysis from this pilot study indicates that a treatment with CsA plus steroids in patients with COVID-19 with moderate to severe pneumonia is associated with two times or around 200% higher probability of improvement and survival, compared to steroids alone.
- Such encouraging outcomes definitely warrant further investigations on the use of CsA in moderate to severe COVID-19 patients, while CsA's tolerability may be further improved by Eyesiu's nanotechnology and readily scaled up to widespread use.
| "About PEA, and why to use it as food supplement to help maintain and support a healthy lifestyle:" |
Biological mechanism fully characterised:
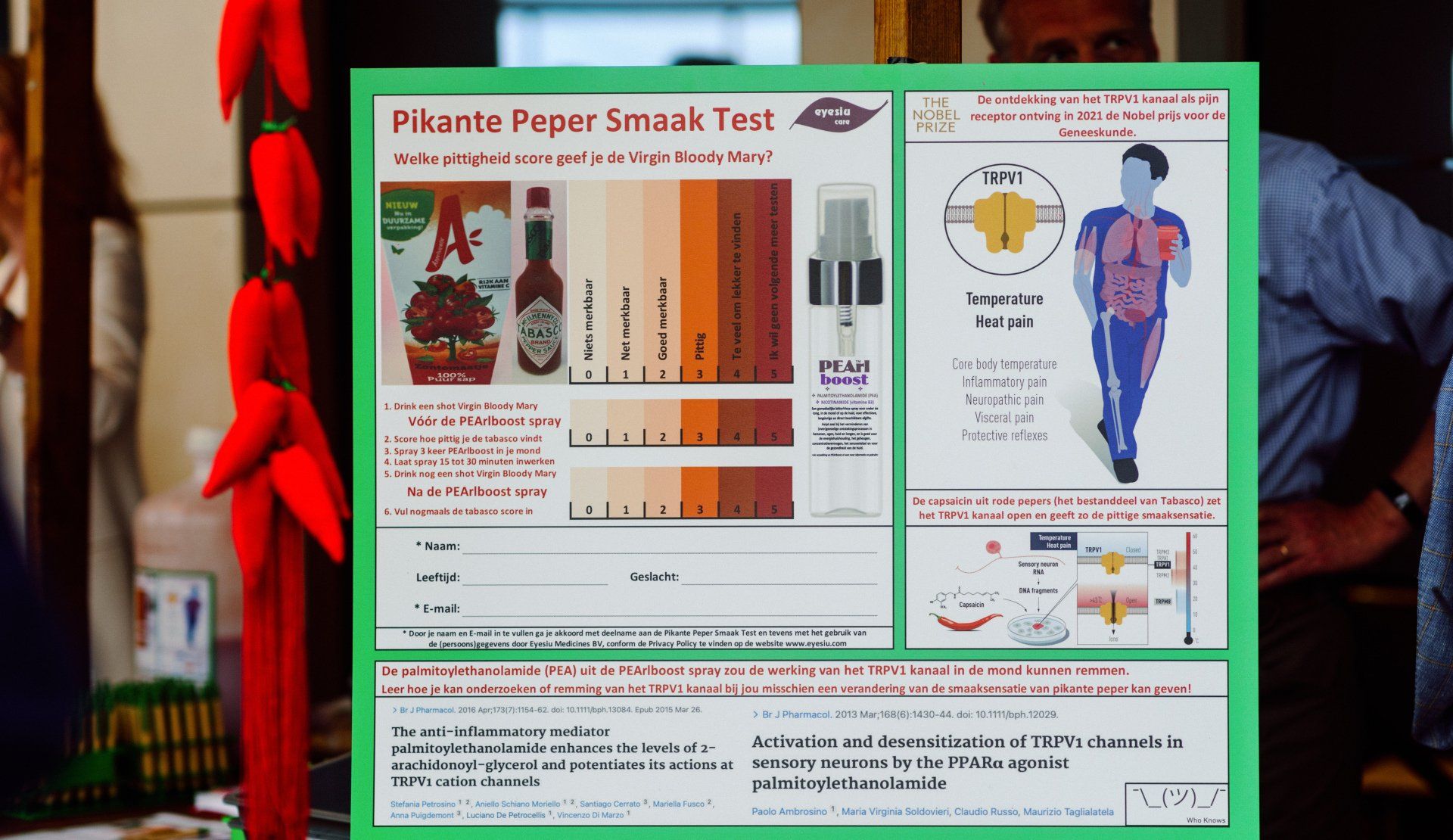
PEA is an endogenous (body-own) fatty acid amide with a wide range of biological functions involved in the body’s response to help reduce chronic pain and inflammation. PEA is clinically and scientifically proven to directly activate PPAR-α, GPR55 and GPR119, and indirectly CB1 & CB2 receptors and TRPV1 channels, collectively known as the “entourage effect” (Petrosino et al (2017)).
Supporting role in many human and animal uses:
There are numerous scientific publications on the physiologically supportive role of PEA on the immune system and the natural cellular response in conditions involving chronic pain complaints (neuropathic, musculoskeletal, fibromyalgia, endometriosis, pelvic pain, migraine); epilepsy, amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson's disease (PD), Alzheimer's disease (AD), autism, sleep disturbance, depression, stroke; glaucoma, diabetic retinopathy (DR); geographical tongue, psoriasis, atopic dermatitis, rosacea, acne; hay fever, cough, asthma, idiopathic pulmonary fibrosis and cystic fibrosis, COPD, colds and flu. These publications are summarised in this extensive blog about PEA.
PEA has been freely available for consumers as a food supplement for many years:
Physiological PEA levels can become reduced, yet never depleted, and since PEA is also highly present in food sources, such as from soybean, egg yolk and peanuts, PEA is regarded as a "vitamin" and marketed as food supplement, freely available for consumers. PEA is therefore readily available in many (web)shops and drugstores for all interested consumers. These PEA supplements (capsules, powders, liposomes, creams, spray) support the consumer to keep the brain, eyes, lungs, skin and hair healthy and free of pain. The capsules, powders and liposomes containing PEA must be swallowed several times a day for several weeks in order to observe efficacy. Instead, the PEArlboost spray solution, when applied under the tongue or on the skin, provides a fast and long-lasting effect, at a low dose in an easy to administer form.